Taylorjm
Active Member
So here's what I have. My well has an iron content between 2-4ppm the two times I had it tested. For the first couple years I used big blue filters and water softener for iron removal, but then I installed a hydrogen peroxide injection system and carbon backwashing filter. So the water comes in, has the peroxide injected, goes to the pressure tank. Then to the backwashing carbon filter. Then to a 4x20 big blue 5 micron filter. This filter would originally turn bright orange right away and the clear housing (yes I know clear housings are bad) would be coated with iron residue. Then it goes to a water softener, then to another 4x20 big blue 5 micron.
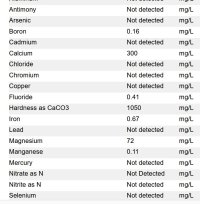
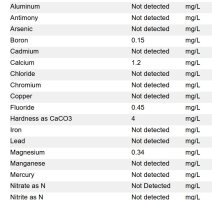
So after installing the peroxide and carbon backwashing filter, the 4x20 would take a couple months to turn a little bit orange and the housing wouldn't have any orange on the plastic. Then it goes to the water softener, but the second 4x20 has big streaks of iron stains on the inside of the housing. I don't know where the iron stains are coming from but there's only maybe 10' of copper lines, then the filter after the softener, so it seems like it has to be coming from the softener. So can a softener put iron back in the water? I ran rust out on backwashing a few times in a row to try and clean it out so i'm not sure what to do now. The picture shows some of the orange streaks but it gets a lot worse right away. I attached the lab tests for untreated and after treatment. So the hardness and iron are gone. This test only showed the iron at 0.67ppm but previous lab tests were higher. The carabon backwashing filter isn't in the pictures.
So after installing the peroxide and carbon backwashing filter, the 4x20 would take a couple months to turn a little bit orange and the housing wouldn't have any orange on the plastic. Then it goes to the water softener, but the second 4x20 has big streaks of iron stains on the inside of the housing. I don't know where the iron stains are coming from but there's only maybe 10' of copper lines, then the filter after the softener, so it seems like it has to be coming from the softener. So can a softener put iron back in the water? I ran rust out on backwashing a few times in a row to try and clean it out so i'm not sure what to do now. The picture shows some of the orange streaks but it gets a lot worse right away. I attached the lab tests for untreated and after treatment. So the hardness and iron are gone. This test only showed the iron at 0.67ppm but previous lab tests were higher. The carabon backwashing filter isn't in the pictures.
Attachments
Last edited: