hydrosean
New Member
I'm really impressed with the on the ground knowledge in these forums. We have kind of an urgent situation and if any of you offer consulting, we would greatly appreciate some closer support than these forums also to help us to get our filters working this week as our family business is hanging on the brink, hinging on getting these filters to work! Interested in anyone's ideas but if you can also consult closer with us, please email me at sean@mortonswarmsprings.com.
We operate a hot springs pools business in northern California and recently installed Katalox Light filters to filter iron and manganese from our hot spring water in order to chlorinate the pools and not have these metals precipitate in the pools and discolor the water brown, green and/or yellow. We are having trouble with getting these new filters to work properly, particularly with the manganese.
Water and filter specs:
Water Source #1 - Hot Springs Source (to be filtered for pools)
108.7 F (and 107 F entering pools after filters)
pH 8.0
TDS 830 ppm
Alkalinity (as CaCO3) 530 ppm
Hardness (as CaCO3) 31 ppm
Bicarbonate 650 ppm
Sulfate 0.57 ppm
Nitrate 0 ppm
Sodium 280 ppm
Chloride 130 ppm
Iron 0.140 ppm currently (has been as high as 0.380 ppm)
Manganese 0.050 ppm currently
Water Source #2 - Warm Springs Source (used for drinking water and to backflush tanks)
91 F
pH 7.8
TDS 480 ppm
Alkalinity (as CaCO3) 250 ppm
Hardness (as CaCO3) 28 ppm
Bicarbonate 310 ppm
Sulfate < 0.5 ppm
Nitrate 0 ppm
Sodium 130 ppm
Chloride 98 ppm
Iron 0.080 ppm (has been as high as 0.440 ppm)
Manganese 0.140 ppm
Filters:
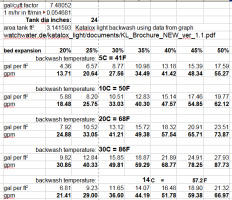
We had a grey color tint to the water exiting the tanks for a number of weeks (especially visible the deeper the pool gets, until its black in the deep end and you couldn't see the bottom!). Then we were advised by Watch Water (who makes KL) that the hot water was not providing enough bed expansion to clean the dust off of the media at 107 F so we switched to using the warm source to backflush at 85 F, which would require less flow to achieve the expansion needed. We were also losing a decent amount of media out of the drain line when backflushing. We ended up having to replace a piston in the control valve of one tank because it got media in it and ground the gears on it. I wondered if the losing of media was because we did not have enough freeboard (40% is recommended and we have 33%). We had to push it on how much media we put in that size tank because of our height restrictions where the tanks are and the need for the 70 gpm total flow. There were DLFCs installed which were keeping the tanks from losing potentially more media but Watch Water recommended we remove those which we did to ensure we get enough options for maximal flow with the warmer water (we could restrict flow on the drain line ourselves using a gate valve and flowmeter if necessary) and instead install top distributor baskets to keep the media from coming through at these higher flow rates (which we did). They recommended 65 gpm with the hot water and we could not achieve this so we switched to using the warm source at average 85 F at about 45 gpm which at that temp they thought would be adequate. They told us to install top distributor baskets of 0.5 mm (#35 mesh) to reduce the chance of clogging over time but will still keep most of the media from going to the drain, but all that was available for our control valve was 0.25-0.3 mm (#50-60 mesh). We are looking into a way to put wire spacers between each mesh opening to modify each basket to be the larger opening size they recommended because we have not found any other available with the correct mesh size. Of course I've seen on the forums what happens to these baskets clogging with oxidized iron over time but I also don't want to lose any more media. Maybe just a maintenance thing over time and to monitor backwash flow to see if it goes down over time. Anyway, with the new top distributors, with the DLFCs removed, and using the warm source with chlorinated 85 F water for 90 minutes backflush each, we finally were able to rinse all of the dust off and the filters ran clear with no grey tint from there.
Now we finally were working on whether the iron and manganese was being filtered or not. Turned out at that point that the iron and manganese was not being filtered at all. But we stopped using chlorine and then the iron started getting filtered completely but not the manganese. Every time we added an oxidant like chlorine or hydrogen peroxide, the iron would stop getting filtered (same amount going in and coming out) and the manganese would actually jump up to being up to 5-9 times coming out of the filters compared to what was going in to the filters. After reading through all of these forums, I'm realizing that we need to be backflushing a lot more than we are, especially now that we are using a better flow rate and cooler water. My experience with Greensand Plus using a mix of the warm and hot sources was that backflushing caused the pools to go green for a week but that may have been related to the ancient bed of gravel that was full of iron in those old tanks that we finally removed. So I was backflush averse, you could say. I actually ran a whole summer season without backflushing once and it ran perfectly the whole time. But this is a different situation it seems, maybe the temperature and bubbles are different now too, see below. So anyway, starting today I'm now backflushing daily for 20 minutes per tank with a 5 minute rinse at 45 gpm from our 85 F warm source.
The other factor I'm considering is the high bicarbonate in our hot water being filtered. This produces quite a bit of bubbles, especially since it takes a good 2.5 minutes to travel from the hot source well (which is also 400 feet deep drawing from a 2" pipe) so there is a lot of time for CO2 to be released in the pipe as it travels towards the pools. We are wondering how much of a factor the bubbles are playing in creating channeling in the media. Do they find and help create the channels and make them deeper along with the water and do the oxidants contribute in making those bubbles more vigorous somehow and/or stir up the oxidized minerals caught along them by the media helping them to break through? Are we getting enough bed expansion to adequately clean the media and disrupt all of the channels? Our tanks are black on the outside but we are going to try and sand the paint off and shine a light to see if we can see the media movement during backflushing, like I saw described on these forums. Assuming the grey tint went away because there was finally enough bed expansion and that is enough - we are hoping.
So our idea is that the more frequent backflushing will minimize possible channeling. And we are also going to try to create some kind of degassing chamber before the KL tanks by using a large 8" stand pipe where water can flow through from the bottom to exit the side around the middle and bubble up to a top release area where a valve can bleed excess CO2. To test the idea we can try this out while the water is running as a concept before looking for a more permanent solution because water would just blow out the top once you turned the water off downstream, so this is not a permanent solution.
Finally, would an aerator be helpful with the bubbles? Would hydrogen peroxide be helpful with the bubbles instead of using chlorine? We're also considering using hydrogen peroxide instead of chlorine for the backflush at least a few times to use its oxygen bubbling effects and its ability to potentially reduce and clean out any built up iron or manganese that has hardened inside the media, if this makes sense?
Also wondering if we need to add sodium hydroxide to bring pH up to 8.5 for manganese filtering like the Watch Water KL website says? pH was above 10 coming out of the tanks to start then came down to 8.4 for awhile, just as was expected, and now it is the same coming out as it is going in - pH 8.0
Thank you everyone for reading and considering. We are going to close for most of the weekdays next week so we can test some of these things out. Our family business is hanging in the balance here and we are losing a lot of revenue because of these disruptions. It's a long story why we are having to test all of this out while being open but circumstances beyond our control made it impossible to test earlier like we had planned. Thanks again for your help!
Sean
We operate a hot springs pools business in northern California and recently installed Katalox Light filters to filter iron and manganese from our hot spring water in order to chlorinate the pools and not have these metals precipitate in the pools and discolor the water brown, green and/or yellow. We are having trouble with getting these new filters to work properly, particularly with the manganese.
Water and filter specs:
Water Source #1 - Hot Springs Source (to be filtered for pools)
108.7 F (and 107 F entering pools after filters)
pH 8.0
TDS 830 ppm
Alkalinity (as CaCO3) 530 ppm
Hardness (as CaCO3) 31 ppm
Bicarbonate 650 ppm
Sulfate 0.57 ppm
Nitrate 0 ppm
Sodium 280 ppm
Chloride 130 ppm
Iron 0.140 ppm currently (has been as high as 0.380 ppm)
Manganese 0.050 ppm currently
Water Source #2 - Warm Springs Source (used for drinking water and to backflush tanks)
91 F
pH 7.8
TDS 480 ppm
Alkalinity (as CaCO3) 250 ppm
Hardness (as CaCO3) 28 ppm
Bicarbonate 310 ppm
Sulfate < 0.5 ppm
Nitrate 0 ppm
Sodium 130 ppm
Chloride 98 ppm
Iron 0.080 ppm (has been as high as 0.440 ppm)
Manganese 0.140 ppm
Filters:
| TANK & CONTROLLER MODEL: Pentair 24X65 COMP 4"T, Clack WS 1.5 El Flow Controller |
| TEMP LIMITS - TANK = 120 F, CONTROLLER = 110 F, MEDIA = 140 F |
| TANK VOLUME & WEIGHT: 13.36 ft3, 109 LB |
| MEDIA: 8 ft3 of Katalox Light media, 528 LBS per tank |
| TOTAL DRY WEIGHT = 637 LBS per tank |
| MEDIA BED DEPTH: 31" |
| GRAVEL/QUARTZ BED: 1 ft3 |
| FREEBOARD: 4.36 ft3 (33% volume, 40% is recommended) |
| NUMBER OF TANKS: 4 |
| NORMAL SERVICE FLOW RATE: 17.5 gpm per tank (70 gpm total, set to not exceed this flow rate for normal service) |
| NORMAL SERVICE FLOW RATE: 5.6 gpm / ft2 (divide by 3.14 for 24" tank) |
| MAXIMUM FLOW RATE: 21 gpm per tank (82 gpm total) = 6.7 gpm / ft2 |
| INLET pH: 8.0 |
| EXIT pH: 8.0 |
| CHLORINE REGENERATION: YES, CONTINUOUS at 1 ppm exiting tank |
| BACKFLUSH FLOW RATE: 45 gpm |
| BACKFLUSH FREQUENCY / SCHEDULE: |
| Moving to daily now, 20 minutes backflush with chlorine @ 45 gpm, 5 minutes rinse @ 20 gpm |
| Using Warm Source @ 85 F (30 gpm from well pump at 91 F and 15 gpm or so from holding tank at lower temp) |
We had a grey color tint to the water exiting the tanks for a number of weeks (especially visible the deeper the pool gets, until its black in the deep end and you couldn't see the bottom!). Then we were advised by Watch Water (who makes KL) that the hot water was not providing enough bed expansion to clean the dust off of the media at 107 F so we switched to using the warm source to backflush at 85 F, which would require less flow to achieve the expansion needed. We were also losing a decent amount of media out of the drain line when backflushing. We ended up having to replace a piston in the control valve of one tank because it got media in it and ground the gears on it. I wondered if the losing of media was because we did not have enough freeboard (40% is recommended and we have 33%). We had to push it on how much media we put in that size tank because of our height restrictions where the tanks are and the need for the 70 gpm total flow. There were DLFCs installed which were keeping the tanks from losing potentially more media but Watch Water recommended we remove those which we did to ensure we get enough options for maximal flow with the warmer water (we could restrict flow on the drain line ourselves using a gate valve and flowmeter if necessary) and instead install top distributor baskets to keep the media from coming through at these higher flow rates (which we did). They recommended 65 gpm with the hot water and we could not achieve this so we switched to using the warm source at average 85 F at about 45 gpm which at that temp they thought would be adequate. They told us to install top distributor baskets of 0.5 mm (#35 mesh) to reduce the chance of clogging over time but will still keep most of the media from going to the drain, but all that was available for our control valve was 0.25-0.3 mm (#50-60 mesh). We are looking into a way to put wire spacers between each mesh opening to modify each basket to be the larger opening size they recommended because we have not found any other available with the correct mesh size. Of course I've seen on the forums what happens to these baskets clogging with oxidized iron over time but I also don't want to lose any more media. Maybe just a maintenance thing over time and to monitor backwash flow to see if it goes down over time. Anyway, with the new top distributors, with the DLFCs removed, and using the warm source with chlorinated 85 F water for 90 minutes backflush each, we finally were able to rinse all of the dust off and the filters ran clear with no grey tint from there.
Now we finally were working on whether the iron and manganese was being filtered or not. Turned out at that point that the iron and manganese was not being filtered at all. But we stopped using chlorine and then the iron started getting filtered completely but not the manganese. Every time we added an oxidant like chlorine or hydrogen peroxide, the iron would stop getting filtered (same amount going in and coming out) and the manganese would actually jump up to being up to 5-9 times coming out of the filters compared to what was going in to the filters. After reading through all of these forums, I'm realizing that we need to be backflushing a lot more than we are, especially now that we are using a better flow rate and cooler water. My experience with Greensand Plus using a mix of the warm and hot sources was that backflushing caused the pools to go green for a week but that may have been related to the ancient bed of gravel that was full of iron in those old tanks that we finally removed. So I was backflush averse, you could say. I actually ran a whole summer season without backflushing once and it ran perfectly the whole time. But this is a different situation it seems, maybe the temperature and bubbles are different now too, see below. So anyway, starting today I'm now backflushing daily for 20 minutes per tank with a 5 minute rinse at 45 gpm from our 85 F warm source.
The other factor I'm considering is the high bicarbonate in our hot water being filtered. This produces quite a bit of bubbles, especially since it takes a good 2.5 minutes to travel from the hot source well (which is also 400 feet deep drawing from a 2" pipe) so there is a lot of time for CO2 to be released in the pipe as it travels towards the pools. We are wondering how much of a factor the bubbles are playing in creating channeling in the media. Do they find and help create the channels and make them deeper along with the water and do the oxidants contribute in making those bubbles more vigorous somehow and/or stir up the oxidized minerals caught along them by the media helping them to break through? Are we getting enough bed expansion to adequately clean the media and disrupt all of the channels? Our tanks are black on the outside but we are going to try and sand the paint off and shine a light to see if we can see the media movement during backflushing, like I saw described on these forums. Assuming the grey tint went away because there was finally enough bed expansion and that is enough - we are hoping.
So our idea is that the more frequent backflushing will minimize possible channeling. And we are also going to try to create some kind of degassing chamber before the KL tanks by using a large 8" stand pipe where water can flow through from the bottom to exit the side around the middle and bubble up to a top release area where a valve can bleed excess CO2. To test the idea we can try this out while the water is running as a concept before looking for a more permanent solution because water would just blow out the top once you turned the water off downstream, so this is not a permanent solution.
Finally, would an aerator be helpful with the bubbles? Would hydrogen peroxide be helpful with the bubbles instead of using chlorine? We're also considering using hydrogen peroxide instead of chlorine for the backflush at least a few times to use its oxygen bubbling effects and its ability to potentially reduce and clean out any built up iron or manganese that has hardened inside the media, if this makes sense?
Also wondering if we need to add sodium hydroxide to bring pH up to 8.5 for manganese filtering like the Watch Water KL website says? pH was above 10 coming out of the tanks to start then came down to 8.4 for awhile, just as was expected, and now it is the same coming out as it is going in - pH 8.0
Thank you everyone for reading and considering. We are going to close for most of the weekdays next week so we can test some of these things out. Our family business is hanging in the balance here and we are losing a lot of revenue because of these disruptions. It's a long story why we are having to test all of this out while being open but circumstances beyond our control made it impossible to test earlier like we had planned. Thanks again for your help!
Sean